Merck Covid Drug
An independent board of experts. Merck announced Friday it will ask the Food and Drug Administration to grant emergency use authorization for an anti-coronavirus drug that reduced hospitalizations by nearly 50 in clinical trials.
A simple pill to treat the coronavirus has been sought since the start of the pandemic and Fridays announcement was.

Merck covid drug. The vaccines cannot give you COVID-19. Government 17 million doses of an oral antiviral treatment for COVID-19. They will help keep you from getting COVID-19.
Laboratory studies show that Merck Cos MRKN experimental oral COVID-19 antiviral drug molnupiravir is likely to. Pharmaceutical giant Merck announced promising results from a study of a new antiviral drug. Pfizer said its latest mid-to-late-stage trial will enroll.
Fewer subjects discontinued study therapy due to an adverse event in the molnupiravir group 13 compared to the placebo group 34. Laboratory studies show that Merck Cos experimental oral COVID-19 antiviral drug molnupiravir is likely to be effective against known variants of the coronavirus including the dominant. The drug is currently being evaluated in a.
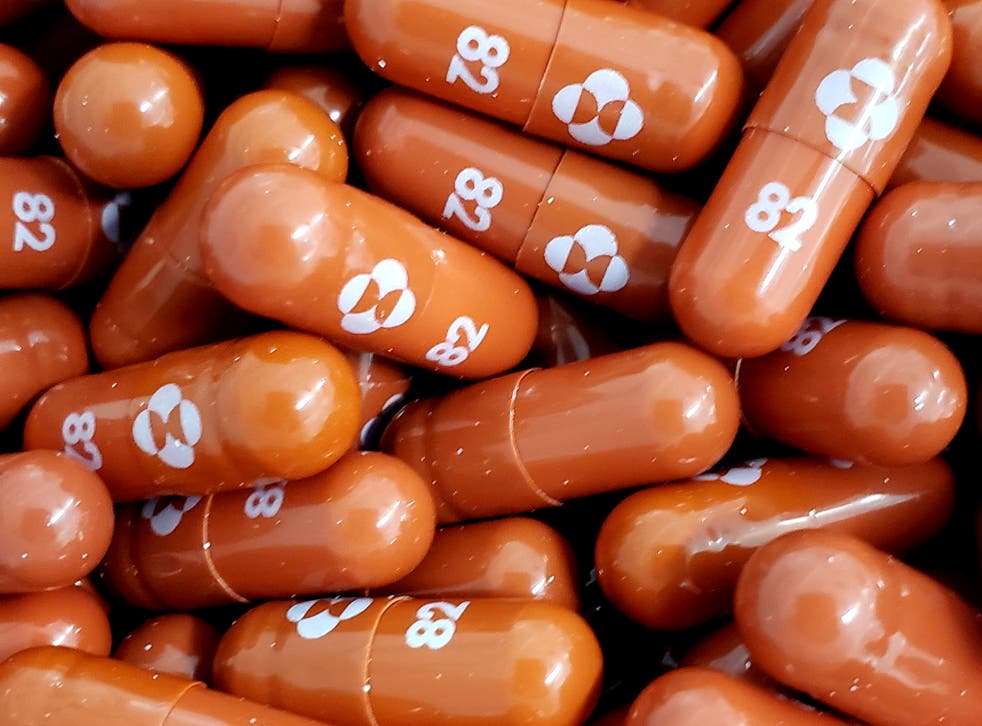
Merck announced encouraging results form a study of its COVID-19 antiviral drug molnupiravir. Merck will seek emergency use authorization for its Covid oral antiviral treatment for Covid-19 citing compelling results in trials. Mercks promising experimental Covid-19 drug raises hopes for pill to fight virus.
US pharmaceutical company Merck said on Friday it will seek authorisation of its oral drug molnupiravir for COVID-19 after it was shown to reduce the chance newly infected patients were hospitalised by 50 per cent. Pfizer Merck launch new trials of oral COVID-19 drugs. About Mercks Efforts to Enable Access to Molnupiravir if it is Granted EUA or Approval.
Mel EvansAP The pharmaceutical giant Merck on Friday reported good news for people sick. An experimental COVID-19 treatment pill called molnupiravir being developed by Merck Co. Experts laud Mercks Covid-19 drug molnupiravir.
Reuters -Pfizer Inc and Merck Co Inc announced on Wednesday new trials of their experimental oral antiviral drugs for COVID-19 as the race to develop an easy-to-administer treatment for the potentially fatal illness heats up. But FDA regulators may consider authorizing it for broader use in vaccinated patients who get breakthrough COVID-19 symptoms. And Ridgeback Biotherapeutics LP is seen in this undated handout photo released by Merck Co.
Ad Safety is CDCs top priority and vaccination is a safer way to help build protection. Merck and its partner Ridgeback Biotherapeutics said early results showed patients who received the drug called molnupiravir within five days of COVID-19 symptoms had about half the rate of. In an early look at the data from a Phase 3 study the company said the drug lowered the risk of.
Merck only studied its drug in people who were not vaccinated. If those promising preliminary results hold the new drug could help fill a significant gap in the. Molnupiravir reduced COVID hospitalizations or death by 50 in a trial involving 775 volunteers.
Merck and closely held Ridgeback Biotherapeutics said Friday their oral drug molnupiravir reduced the risk of hospitalization or death by about 50 in patients with mild to moderate Covid-19. Merck announced Friday that an experimental pill it developed to treat covid-19 reduced the risk of hospitalization and death by nearly half in a clinical trial. Merck and Ridgeback Biotherapeutics said Friday theyve developed a drug that reduces the risk of hospitalization or death by around 50 for patients with mild or moderate cases of Covid.
The drug molnupiravir cut the risk of hospitalization or. Merck announced that its new antiviral drug reduced the risk of hospitalization or death of COVID-19 patients by 50 in a late-stage trial. All you need to know The experimental drug molnupiravir significantly reduced the risk of hospitalization or death when administered to high-risk.
Andrew Pekosz of Johns Hopkins University predicted vaccines and antiviral drugs would ultimately be used together to protect against the worst effects of. Merck is reporting that Molnupiravir a new Covid-19 drug reduces the risk of hospitalization and death. Merck and Ridgeback Biotherapeutics announced on Friday early result that indicate their experimental oral antiviral drug molnupiravir might halve the risk of death or hospitalization from Covid-19.
The drug maker Merck said on Friday that its pill to treat Covid-19 was shown in a key clinical trial to halve the risk of hospitalization or death when given to high-risk people early in their. Mercks Little Brown Pill Could Transform the Fight Against Covid The antiviral drug molnupiravir still in clinical trials would give doctors an important new treatment and a weapon against. Merck Says Research Shows Its COVID-19 Pill Works Against Variants.
Drugmaker Merck reached an approximately 12 billion deal to supply the US. Similarly the incidence of drug-related adverse events was also comparable 12 and 11 respectively.

Merck Mrk Molnupiravir Pill Could Change The Fight Against Covid Bloomberg

Three Pharma Cos In Race To Find Covid 19 Treatment Pill Results Likely By 2021 End Businesstoday

From Pfizer To Shionogi Drugmakers Race For Coronavirus Pills Nikkei Asia

A Daily Pill To Treat Covid Could Be Just Months Away Scientists Say














0 Response to "Merck Covid Drug"
Post a Comment